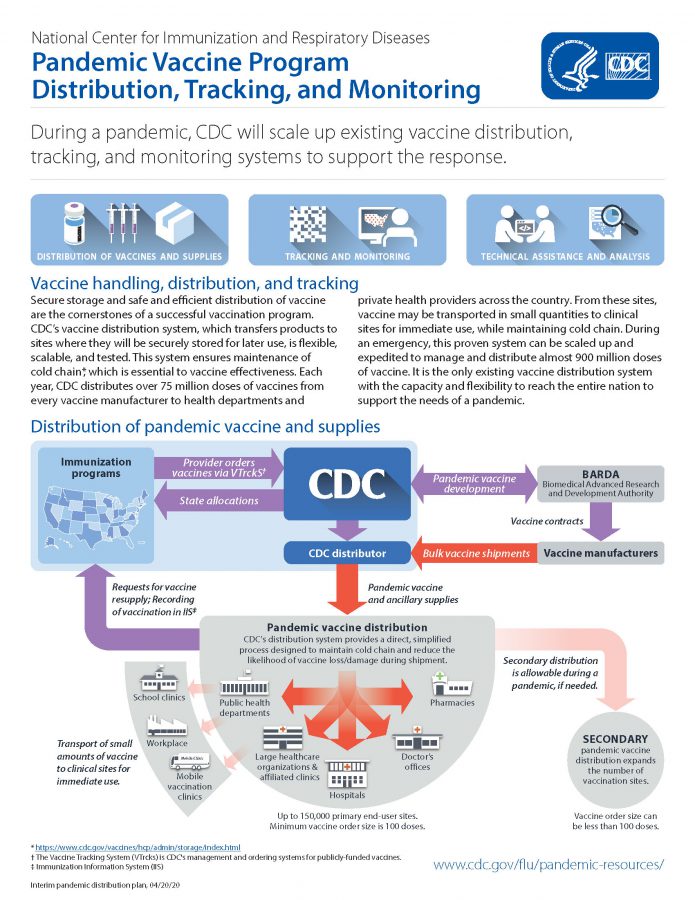
Department of Health and Human Services Centers for Disease Control and Prevention During a pandemic CDC will scale up existing vaccine distribution tracking and monitoring systems to support the response. Use of influenza A H1N1 2009 monovalent vaccine.
 The Benefits Of Flu Vaccination 2019 2020
The Benefits Of Flu Vaccination 2019 2020
Information for Healthcare Professionals about the Screening Checklist for Contraindications to Inactivated Injectable Influenza Vaccination IIV or RIV.

Influenza vaccine cdc pdf. The influenza biological product pages provide guidance on the use of influenza vaccines that are publicly-funded in BC for the 202021 season. Pregnant women children aged 659. Status of influenza AH5 candidate vaccine virus development Candidate vaccine viruses Clade Institution Available AViet Nam12032004 CDC-RG.
Supplemental influenza vaccine doses 2020. Influenza vaccination programmes in order to reduce morbidity and mortality due to influenza. Lisa Grohskopf Flu vaccine is recommended for all children 6 months of age and older.
It replaced the Fluzone Intradermal Trivalent Vaccine. National Center for Immunization and Respiratory Diseases Pandemic Vaccine Program Distribution Tracking and Monitoring US. Due to the high mutation rate of the virus a particular vaccine formulation is effective for at most about a year.
See httpwwwcdcgovflupdfprotectvaccineinfluenza-vaccines-table-2015-16pdf for the complete. 11062021 Flu Vaccine for 2020-2021 Season. Clinician Outreach and Communication Activity COCA Webinar Thursday August 20 2020.
PDF - 37237 KB. Enfermedades Centers for Disease Control and Prevention CDC recomiendan que todas las personas de 6 meses de edad y mayores se vacunen cada temporada contra la gripe. Continuing Education Online system at httpstceols.
Symptoms should resolve quickly but speak to your. Flu Shot Side Effects. For 2020-2021 CDC recommends use of any licensed age-appropriate flu vaccine as an option for vaccination this season.
Influenza vaccine contains 15 g of each antigen per 05 ml dose of the three virus strains usually two type A and one type B that are likely to circulate during the upcoming influenza season CDC 1999. Intradermal Quadrivalent vaccine is a newly licensed influenza vaccine for the 2015-2016 season and is licensed for persons ages 18-64 years. Prevention and Control of Influenza with Vaccines.
In a nutshell Nowaks presentation focused on how to use the media to create fear and anxiety to promote vaccination and increase vaccine uptake in the US. SJRG-161052 1 CDC and SJCRH Yes AViet Nam11942004 NIBRG-14 1 NIBSC Yes ACambodiaR04050502007 NIBRG-88. Side effects are usually minor.
1 8 httpwwwcdcgovmmwrpdfrrrr5810pdf. MMWR April 30 2010 5916485-486 Licensure of a High-Dose Inactivated Influenza Vaccine for Persons Aged 65 Years or Older Fluzone High-Dose and Guidance for Use United States 2010. Nios de 6 meses a 8 aos de edad pueden necesitar 2 dosis durante una sola temporada de gripe.
These include soreness at the site of the shot headache fever nausea and muscle aches according to the Centers for Disease Control CDC. Injectable flu vaccines or flu shots live attenuated influenza vaccines or nasal spray. Children who are 6 months through 8 years old may need two doses of flu vaccine to be fully protected if theyre getting vaccinated for the first time or have previously only received one dose of vaccine.
93 million doses Funding to support operational costs associated with planning and implementation of expanded influenza vaccination program Supplemental vaccine doses to be allocated among the awardees Strong recommendation for awardee partnerships with Community Health Centers CHCs. 2020-2021 Influenza Vaccination Recommendations and Clinical Guidance during the COVID-19 Pandemic. Recommendations of ACIP 2010 Print version cono de pdf 118 MB 36 pginas See page 1.
TETRA FLUVIRAL AGRIFLU FLUAD. 24042019 Nowak gave the presentation at the National Influenza Vaccine Summit in 2004 co-sponsored by the CDC and the American Medical Association AMA. Millions of Americans receive the flu vaccine each year either by choice or because they are compelled to do so by their employer.
All continuing education for COCA Calls are issued online through the CDC Training. WHO recommends that countries consider influenza vaccination for persons at high risk of death or serious illness from influenza and has recognized the following groups to be at increased risk. Todos los dems necesitan solo 1 dosis cada temporada de gripe.
Routine influenza vaccination of health-care personnel HCP every influenza season can reduce influenza-related illness and its potentially serious c. The intended use of these vaccines by age group of recipient for the 202021. Recommendations of the Advisory Committee on Immunization Practices ACIP 2009.
12032014 Centers for Disease Control and Prevention. Groups Recommended for Vaccination Routine annual influenza vaccination is recommended for all persons 6 months of age who do not have contraindications While vaccination is recommended for everyone in this age group there are some for whom it is particularly important People aged 6 months who are at high risk of complications and. Established benefits of influenza vaccination for the majority of people who have a history of GBS and who are at high risk for severe complications from influenza justify yearly vaccination.
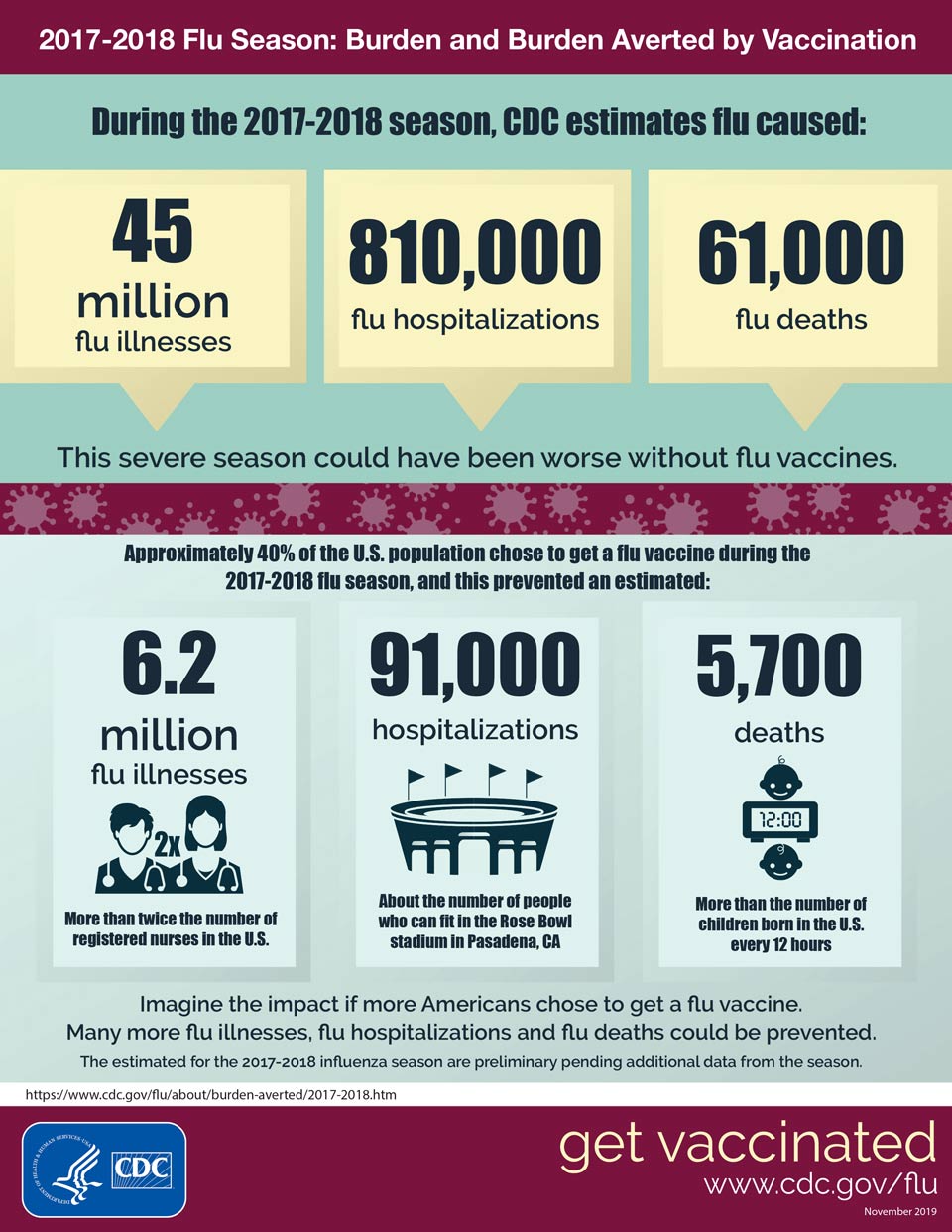 Flu Burden And Flu Burden Averted By Vaccination Infographic Cdc
Flu Burden And Flu Burden Averted By Vaccination Infographic Cdc
 Interim Updated Planning Guidance On Allocating And Targeting Pandemic Influenza Vaccine During An Influenza Pandemic Pandemic Influenza Flu Cdc
Interim Updated Planning Guidance On Allocating And Targeting Pandemic Influenza Vaccine During An Influenza Pandemic Pandemic Influenza Flu Cdc
Https Www Cdc Gov Flu Pdf Professionals Vaccination Make Strong Vaccine Rec 65 2020 Pdf
Https Www Cdc Gov Vaccines Hcp Adults Downloads Fs Influenza Hcp Pdf
Arsip Blog
-
▼
2021
(1509)
-
▼
Mei
(272)
- How Long Does It Take To Get Covid Test Results Fr...
- Cara Download Sertifikat Vaksin 2
- Beli Vaksin Pfizer Di Indonesia
- Vaksin Covid Yang Bagus Merk Apa
- Download Sertifikat Vaksin Covid Online
- What Is The Rt Pcr Covid Test
- Sertifikat Vaksin Di Kaki
- How Long Should I Wait To Get A Covid Test After B...
- Do You Need An Appointment For Covid Testing
- Entry Requirements For Greece Covid
- Covid-19 Vaccine Awareness Quotes
- Rabies Shot Cost For Dog
- Covid Testing Results Time Adelaide
- What Are The Restrictions In Utah Regarding The Co...
- Will Sa Lockdown Be Further Extended
- Vaksin Tetanus
- Where To Get A Covid Test In Melbourne
- Cara Memperbaiki Nama Yang Salah Di Sertifikat Vaksin
- Rabies Vaccine Schedule Missed Dose
- Cdc Covid Travel Guidelines 2021
- Covid-19 Travel Restrictions By State See Testing ...
- What Are The Covid Rules In Wales For Funerals
- Influenza Vaccine Cdc Pdf
- Cara Download Sertifikat Vaksin Ke 2
- Does San Diego Have Travel Restrictions
- Jika Sertifikat Vaksin Ke 2 Tidak Muncul
- Varicella Vaccine Effectiveness
- Sa Covid Rules Now
- Kenapa Sertifikat Vaksin Belum Muncul
- How Long Does It Take To Get Covid-19 Test Results...
- Where Can I Get Same Day Covid Testing For Travel
- Sertifikat Vaksin Ke 2 Tidak Ada
- How Often Should You Get A Tetanus Shot In Canada
- How Many Cases Of Covid 19 In Selby
- Covid Virus Vaccine Tracker
- Covid Test Jobs Birmingham
- What Are Victoria Covid Restrictions
- Sa Covid Restrictions Contact Number
- What Are The Social Restrictions In Pennsylvania D...
- Covid Testing San Antonio Tx Same Day Results
- Sertifikat Vaksinasi Gotong Royong
- Vaksin Covid Untuk Anak 5 Tahun
- Port Adelaide Drive Through Covid Testing
- Where Can I Get Free Covid Antibody Test
- How Much Does A Rapid Covid 19 Test Cost
- What States Have Travel Restrictions For Covid-19
- Daftar Vaksin Covid Untuk Umum Di Jakarta
- Covid Testing Adelaide Cost
- Sa Pathology Covid Test Results Online
- Covid Rules For Entering Saskatchewan
- Cara Daftar Vaksin Covid Untuk Masyarakat Umum Sur...
- Vaksinasi Covid Tangerang Kota
- Airport Test Covid Brussels
- What Are The Restrictions In Alberta For Covid
- Is Drive Thru Covid Testing Free
- Same Day Results Covid Testing San Antonio Tx
- Sask Covid Restrictions June 2021
- Astrazeneca Vaccine France News
- Cara Vaksin Ayam Yang Bagus
- What Are Latest Covid Rules
- Cara Daftar Vaksin Lansia Surabaya
- Vaksin Covid Untuk Umum Kapan
- Which States Have Travel Bans Due To Covid 19
- Sertifikat Vaksin Namanya Salah
- How To Get Covid Test Results Saskatchewan
- Sertifikat Vaksin Belum Keluar
- San Diego County Covid Business Guidelines
- What Are Current Covid 19 Restrictions In Ireland
- Sertifikat Vaksin Pedulilindungi
- Covid Testing Sites Adelaide Wait Times
- Closest Covid Testing Centres
- How Long Does It Take To Get Covid Test Results Ba...
- Cara Cek Sertifikat Vaksin Ke 2
- What Is The Current Covid Rules In England
- Vaksin Dibuat Dari Apa
- Sertifikat Vaksin Itu Seperti Apa
- Government Paid Covid Testing
- Is The Government Paying For Covid Testing
- How Soon After Traveling Covid Test
- Vaksin Sinovac Untuk Lansia Usia Berapa
- Link Sertifikat Vaksin Hilang
- Is The Blood Test For Covid 19 Accurate
- Vaksin Covid 19 Grand City Surabaya
- Cara Cek Sertifikat Vaksin Yang Hilang
- Vaksin Tangerang Selatan 18 Tahun
- Varicella Vaccine Name
- Vaksin Astrazeneca Adalah Virus Yang Dilemahkan
- Sertifikat Vaksin Belum Muncul Di Pedulilindungi
- Vaksinasi Umum Di Jakarta
- Vaksin Sinovac Atau Astrazeneca
- Vaksin Imunisasi Terbuat Dari Apa
- Download Sertifikat Vaksin Online Tanpa Nomor Hp
- Vaksinasi Covid Kabupaten Tangerang
- Covid 19 Test Same Day Results Miami
- Sertifikat Vaksin Fake
- Sertifikat Vaksin Covid 19 Tahap 2
- Sertifikat Vaksin Mandiri
- Does The Airport Ask For Covid Test
- Anti Rabies Vaccine For Dogs Uk
- Sertifikat Vaksinasi Tidak Muncul
-
▼
Mei
(272)